ar periodic table
Have you ever wondered about the fascinating world of elements? The ar periodic table is a comprehensive guide that categorizes and organizes all known elements based on their atomic number, electron configuration, and other properties. In this article, we will delve into the intricacies of the ar periodic table, exploring its structure, significance, and applications in various fields.
Understanding the Structure of the ar Periodic Table
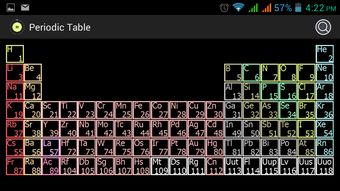
The ar periodic table is divided into several sections, each with its unique characteristics. The table consists of rows called periods and columns called groups. The periods represent the energy levels of the electrons in an atom, while the groups represent the number of valence electrons an element has.
Let’s take a closer look at the structure of the ar periodic table:
| Period | Group | Number of Elements |
|---|---|---|
| 1 | 1 | 2 |
| 2 | 2 | 8 |
| 3 | 3 | 8 |
| 4 | 4 | 18 |
| 5 | 5 | 18 |
| 6 | 6 | 32 |
| 7 | 7 | 32 |
As you can see, the number of elements in each period increases as you move down the table. This is due to the addition of new energy levels in each period. The groups are further divided into s, p, d, and f blocks, which represent the types of orbitals that the valence electrons occupy.
Significance of the ar Periodic Table
The ar periodic table is a powerful tool that helps scientists and researchers understand the properties and behaviors of elements. Here are some key reasons why the ar periodic table is significant:
-
Organizes Elements: The table provides a systematic way to organize and categorize all known elements, making it easier to study and compare their properties.
-
Predicts Properties: By examining the position of an element in the table, scientists can predict its physical and chemical properties, such as melting point, boiling point, and reactivity.
-
Facilitates Discovery: The table has been instrumental in the discovery of new elements, as scientists can predict the properties of elements based on their position in the table.
-
Understands Periodic Trends: The table helps us understand the periodic trends, such as atomic radius, ionization energy, and electronegativity, which are essential in explaining the behavior of elements.
Applications of the ar Periodic Table
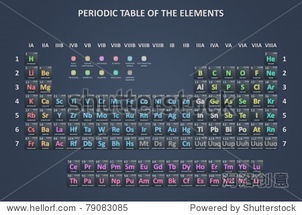
The ar periodic table has numerous applications in various fields, including:
-
Chemistry: Chemists use the table to predict the reactivity of elements, understand the formation of compounds, and design new materials.
-
Physics: Physicists use the table to study the electronic structure of atoms, understand the behavior of electrons, and develop new technologies.
-
Biology: Biologists use the table to understand the composition of living organisms, study the role of elements in biological processes, and develop new drugs.
-
Environmental Science: Environmental scientists use the table to study the distribution of elements in the environment, understand the impact of pollutants, and develop strategies for environmental protection.
Conclusion
The ar periodic table is an invaluable resource that has revolutionized the field of chemistry and other related sciences. By organizing and categorizing all known elements, the table provides a framework for understanding the properties and behaviors of elements. As we continue to explore the universe, the ar periodic table will undoubtedly play a crucial role in unraveling










