Understanding the Concept of Ar Molar Mass
Have you ever wondered what the molar mass of argon (Ar) is and why it’s important? Molar mass is a fundamental concept in chemistry that helps us understand the properties of substances. In this article, we will delve into the molar mass of argon, its significance, and how it is calculated. Let’s embark on this journey of discovery.
What is Molar Mass?
Molar mass is the mass of one mole of a substance. It is expressed in grams per mole (g/mol) and is calculated by summing the atomic masses of all the atoms in a molecule. The atomic mass of an element is the average mass of its atoms, taking into account the natural abundance of its isotopes.
Atomic Mass of Argon
Argon is a noble gas, which means it is a non-reactive element. Its atomic number is 18, and it has three naturally occurring isotopes: Ar-36, Ar-38, and Ar-40. The atomic mass of argon is calculated by taking the weighted average of the atomic masses of these isotopes, considering their natural abundance.
| Isotope | Atomic Mass (amu) | Abundance (%) |
|---|---|---|
| Ar-36 | 35.9675 | 0.000033 |
| Ar-38 | 37.9627 | 0.000629 |
| Ar-40 | 39.9624 | 99.9669 |
Using the table above, we can calculate the atomic mass of argon as follows:
Atomic mass of Ar = (35.9675 脳 0.000033) + (37.9627 脳 0.000629) + (39.9624 脳 0.999669) = 39.948
Calculating the Molar Mass of Argon
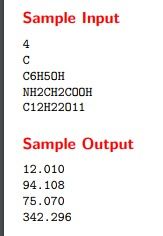
Now that we have the atomic mass of argon, we can calculate its molar mass. The molar mass of a substance is simply the atomic mass of that substance. Therefore, the molar mass of argon is 39.948 g/mol.
Significance of Molar Mass
The molar mass of a substance is crucial for various reasons. It helps us determine the amount of substance present in a given mass, which is essential in chemical reactions and calculations. Additionally, molar mass is used to calculate the density, molarity, and concentration of solutions.
Applications of Molar Mass
Molar mass has numerous applications in various fields. Here are a few examples:
-
In chemistry, molar mass is used to balance chemical equations and determine the stoichiometry of reactions.
-
In pharmaceuticals, molar mass is used to ensure the purity and quality of drugs.
-
In environmental science, molar mass is used to analyze the composition of air and water samples.
-
In food science, molar mass is used to determine the nutritional value of food products.
Conclusion
Understanding the molar mass of argon and other substances is essential in various scientific fields. By calculating the molar mass, we can gain insights into the properties and behavior of substances. So, the next time you encounter the term “molar mass,” remember that it is a fundamental concept that plays a significant role in our daily lives.










